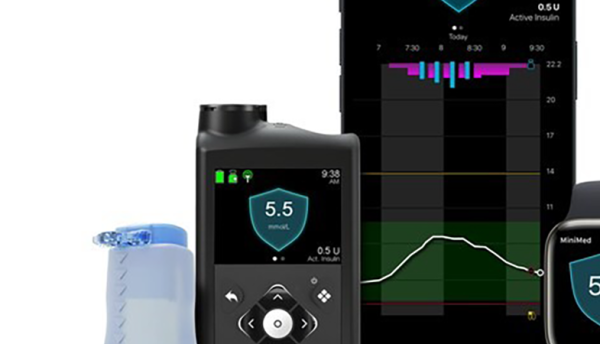
Medtronic, a global leader in healthcare technology, received CE (Conformité Européenne) Mark approval for the MiniMed 780G system with Simplera Sync, a disposable, all-in-one continuous glucose monitor (CGM) requiring no fingersticks or overtape. Simplera Sync features an improved user experience with a simple, two-step insertion process and is half the size of previous Medtronic sensors.
The MiniMed 780G system with Simplera Sync sensor will be available in Europe via limited release in spring 2024. Medtronic will begin the phased commercial launch in Europe in the summer of 2024. Now, the MiniMed 780G system can be used with the Guardian 4 sensor.
The MiniMed 780G system is Medtronic’s most advanced insulin delivery system, automatically adjusting and correcting† glucose levels every five minutes. It’s the world’s only system with a Meal Detection feature that is designed to reduce post-meal hyperglycemia when users occasionally forget to give themselves insulin or underestimate the number of carbs in their snacks or meal. The system, which is available with the world’s only seven-day infusion set, also features one of the lowest glucose target settings (as low as 100 mg/dL) of any automated insulin delivery system. With this ‘treat to target’ approach, the system more closely mirrors the glucose levels of someone not living with diabetes. With both Simplera Sync sensor and the Guardian 4 sensor, no fingersticks are required.
“A challenging aspect of living with diabetes is counting carbohydrates and dosing the right amount of insulin before consuming snacks and meals. Many people underestimate their carbs, which can lead to high blood sugars (hyperglycemia). Prolonged hyperglycemia can lead to serious health problems impacting the eyes, major organs, and even cognitive function, which is particularly concerning in developing children,” Robert Vigersky, M.D., Chief Medical Officer, Medtronic Diabetes, Professor of Medicine, Uniformed Services University of the Health Sciences “With its responsive algorithm, the MiniMed 780G system can help people living with diabetes even when they occasionally forget to bolus or undercount their carbs. The system takes on more of the work involved in diabetes management and helps alleviate mental burden.”
“We’re incredibly proud that the MiniMed 780G system continues to be the most widely used automated insulin delivery system in Europe since we launched it in 2020. Real-world data on over 100,000 users on the system across many geographies and cultures shows that when using recommended settings, the system is delivering an average Time in Range of nearly 80%, raising the bar on what ‘good’ looks like,” said Que Dallara, EVP and President, Medtronic Diabetes. “With the introduction of Simplera Sync sensor, we’re able to offer the proven benefits of our MiniMed 780G system with our newest and most comfortable sensor that can be applied in under 10 seconds.”
The MiniMed 780G system with Simplera Sync sensor is indicated for ages 7+ and compatible with iOS and Android. Simplera Sync sensor is not approved by the FDA and is limited to investigational use in the US.
Simplera Sync sensor is designed to leverage Medtronic’s advanced AID algorithm as part of its MiniMed 780G system while having a similar look and feel as the Simplera CGM. The Simplera CGM for integrated use with the InPen smart insulin pen received CE Mark in September 2023.